Comparative study of ortho-phthalaldehyde and glutaraldehyde for endoscopic disinfection
Release time:
2022-06-13
To study the clinical application effect of o-phthalaldehyde and glutaraldehyde in endoscopic disinfection. Methods from September 2011 to March 2015
After monthly endoscopic examination, 80 cases of endoscopes were divided into different disinfection treatment groups, group A underwent phthalaldehyde disinfection, group B underwent glutaraldehyde disinfection, each.
In 40 cases, the effect was evaluated after disinfection treatment. Results The qualified rate of two groups of endoscopes after disinfection treatment was 1 0 0.0%, and the difference was not statistically significant (P> 0.05).
However, the clinical work efficiency (appointment time, total disinfection) and endoscopic disinfection time of group A were better than those of group B, and the difference was statistically significant (P <0.05). knot
The clinical use of phthalaldehyde to disinfect endoscopes has a significant effect, which can shorten the disinfection time and reduce toxicity, and is worth using.
In recent years, with the improvement and development of clinical medical technology, with
Endoscopic techniques with clinical advantages such as simplicity and small trauma are widely used in clinical disease detection.
and treatment, and achieved significant results. However, due to the high clinical costs of such techniques,
And direct contact with the patient's organs, once the pre-use disinfection treatment is not complete, will be.
Reduce the clinical effect, and even endanger the safety of patients [1]. Therefore, how to improve
The disinfection effect of endoscopes and reducing the cost of clinical use have become the main problems that need to be solved urgently.
The problem. In order to better explore the effect of endoscopic disinfection, this study will be ortho-benzene two in our hospital.
Formaldehyde, glutaraldehyde disinfection treatment of endoscopes as the main object of investigation, the study of benzene two
Clinical application effect of formaldehyde and glutaraldehyde in endoscopic disinfection.
1 Data and methods
1.1 General Information
Endoscopy after endoscopy in our hospital from September 2001 to March 2005
80 cases were divided into different disinfection treatment groups,
A
group of 40 patients,
Male 21 cases, female 19 cases; age 15-79 years, average (45.5±2. 5) years;
Followed by the incision, skin and soft tissue purulent specimens in 2 4 strains, in addition to the urine and
Blood is distributed. Pseudomonas aeruginosa is widely distributed in various places in nature,
At the same time, there are also respiratory tract, skin and other parts of the human body, which are conditional pathogens,
In hospital infection, Pseudomonas aeruginosa belongs to the main pathogenic bacteria, and the strain
Strong ability to colonize, for the prevention of infection caused by Pseudomonas aeruginosa brings great difficulties.
difficult [1]. According to the general distribution of bacterial specimen types, it can be concluded that aeruginosa false
Specimen type of infection caused by single cell bacteria is mainly sputum, the second is pus and secretions.
According to the relevant research results show that the proportion of lung disease has nearly 61. 6%,
The main population were patients with chronic obstructive pulmonary disease and pneumonia, accounting for 22. 6% respectively.
and 2 9.0%, in addition, the postoperative infection rate and incision infection rate are also very high, a total
1 9.0 per cent. According to the occurrence of Pseudomonas aeruginosa in different departments,
Respiratory medicine, intensive care unit and cadre health care disease as the main focus of the department,
The reason for the department patients with low immunity, organ dysfunction, coupled with long-term
A variety of serious diseases, resulting in the proliferation of Pseudomonas aeruginosa, make.
serious consequences. Comprehensive analysis of the above research results, the hospital should increase the relevant
Room disinfection work, follow the principle of aseptic operation, reduce the infection rate of Pseudomonas aeruginosa,
Then according to the characteristics of Pseudomonas aeruginosa infection, the corresponding antibacterial treatment measures were formulated.
According to the bacteria in our hospital room strain separation status, Pseudomonas aeruginosa is a medical
The hospital is more important infection pathogens, in clinical isolates are second only to Escherichia coli.
Hirschia.
Resistance of Pseudomonas aeruginosa to antimicrobial agents mainly produces a variety of antibacterial activities
Enzymes, like
P
-Lactamases, metalloenzymes, and also have the ability to change
Target of action, such as outer mold porin
D
2 is missing. In addition, the efflux
Mechanisms and biofilms may also be important factors leading to the emergence of drug resistance in Pseudomonas aeruginosa
because [2]. Studies have shown [3] that the rate of resistance to sulfonamides is higher than that of amacarcylline.
accounting for 78. 9% and 93. 1%, respectively, in which aminoglycoside antibiotics exist
low resistance, such as amikacin and gentamicin, but nephrotoxic
The reasons for the larger sex lead to a gradual decrease in clinical use, meropenem in vitro live.
The in vitro activity of imipenem and imipenem is relatively good, and the sensitivity rate of imipenem is large.
accounted for about 78. 3%, while the sensitive rate of meropenem accounted for 72. 4%. Carbapenems
In a broad spectrum of highly effective antimicrobial drugs, the drug for the vast majority
P
-Lactam
The enzyme is stable and can be produced without cross-production with third-generation cephalosporins, but the drug
Caution is still required in clinical use to prevent carbapenem resistance due to excessive use.
Pseudomonas aeruginosa appeared. Studies have reported [4] that the use of large amounts of carbapenems
drug in the treatment of infections caused by Pseudomonas aeruginosa prone to meropenem and
Imipenem resistance.
To sum up, clinical measures should be taken to reduce Pseudomonas aeruginosa
Respiratory tract infection and the emergence of drug resistance, at the same time, for the treatment process, it is necessary
The use of antibiotics should be made in time according to the results of drug sensitivity and the change of drug-resistant strains.
adjustment, and take strict disinfection and isolation measures, strict control to prevent infection
Occurrence.
Among them, gastroscope (model 260
Z
) Examine the patient
M
cases, colonoscopy (model
260
A Z I
) Examine 16 patients;
B
Group 4 0 patients, male 2 2 cases, female 18
Cases; Age 16-80 years old, average (45.6±2.6) years old; Among them, gastroscopy
(Model 260
Z
) 25 patients were examined, colonoscopy (model 260
A Z I
1) Inspection
1 5 cases. Two groups of endoscopy in patients with gender, age, the use of endoscopic models such as a
The difference in general data is not statistically significant.
(P
> O. 05), comparable.
1.2 method
Clinical endoscopy disinfection treatment selection of artificial disinfection method, in strict accordance with the health department.
The relevant requirements of the Technical Code of Practice for Cleaning and Disinfection of Endoscopes issued by
Immediately after use, remove the surface dirt with moist gauze, and repeat
The time for water and air supply treatment is
l
〇
s
, and then put the waterproof cap of the endoscope
In commonly used containers sent to the disinfection room for disinfection, endoscopic disinfection in accordance with water washing, acyl washing,
Cleaning, disinfection, flushing and other steps to disinfect, switch between steps will be included in the endoscope.
If there is moisture to dry, blow dry the moisture on the surface of the endoscope at the same time. then will be disinfected after drying
Endoscopes were placed in phthalaldehyde, glutaraldehyde disinfection tank, in the phthalaldehyde
Place 5 in the disinfection tank
m m
Place 10 in glutaraldehyde
m m
, resistant bacteria can be extended
30 ~45
m m
.
After the disinfection of clinical endoscopes, sampling is carried out to facilitate the evaluation of elimination.
toxic effect, Methods:(1) Intracavitary sampling. After endoscope disinfection is completed, use sterile
Inward injection of 0.9% sodium chloride injection by syringe 10.0
m l
, mainly in
The endoscopic biopsy population is then withdrawn at the biopsy exit and sent to the laboratory for examination, 2
h
internal inspection;(2) surface sampling. Wiping the endoscope with the help of a cotton swab shows that
Put the square cotton wool into the sterile test tube and send it for inspection, 2
h
Internal detection.
1.3 assessment items
Evaluate the disinfection effect after the end of the clinical endoscopic sampling test, and record in detail.
The clinical work efficiency and disinfection time of the two groups of disinfectants. endoscopic disinfection effect standard:
If the residual bacterial colony of the endoscope after disinfection is 20 per piece
c f
The following, no pathogenicity fine
Bacteria growth indicates that disinfection is qualified.
1.4 statistical methods
Application
S P S S
18. 0 software for research and analysis of relevant data, measurement.
Data comparison using the "test, counting data comparison using the test, ^ <. o 5
The difference is statistically significant.
2 Results
Evaluation of clinical endoscopic disinfection effect in 2.1
The qualified rate of the two groups of endoscopes after disinfection was 100. 0%, and the difference was not statistically significant.
Meaning (
P
> 0.05). See Table 1.

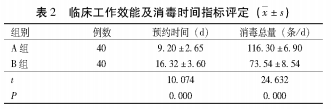
Evaluation of Clinical Work Efficiency and Disinfection Time Index of 2.2
A
group endoscopic disinfection time was 5
m i n
,
B
group endoscopic disinfection time was 10
m i n
,
The difference in clinical disinfection time between the two groups was statistically significant.
(P
3 Discussions
Clinical endoscopy is a kind of human, minimally invasive disease diagnostic equipment, with its
The clinical advantages of small trauma and simple operation are widely used in clinical disease diagnosis and treatment.
In therapy. However, due to the special material and complex structure of endoscopic equipment, the indirect increase in
Difficulty of disinfection treatment after clinical use. Once the endoscope is not completely disinfected, it will be directly
Affect the life safety of patients. At present, the clinical commonly used disinfectant is glutaraldehyde, but this
The class of disinfectants itself is extremely toxic, coupled with the influence of factors such as improper use,
Easily endanger the health of patients and medical staff. And, clinical glutaraldehyde disinfectant
The use time is relatively long, 10~40
m m
, which brings shadow to the repetitive use of endoscopes
reduce the compliance of medical staff endoscopic disinfection, and then increase the hospital cross sex.
Dyeing rate. Therefore, the clinical need to choose a reasonable and effective disinfectant for disinfection. In recent years
In recent years, through continuous research, our hospital has found that the effect of o-phthalaldehyde disinfectant is obvious,
Clinical disinfection rate pass rate and glutaraldehyde disinfectant difference is not statistically significant,.
The difference in disinfection use time between the two groups was statistically significant.
(P
As a result, phthalaldehyde disinfectants are used in endoscopic disinfection in most countries. I...
Hospital through clinical studies also confirmed the efficacy of phthalaldehyde disinfection, but should be exceptionally strong
The adjustment is: endoscopic disinfection treatment requires thorough cleaning, flushing, etc. in the early stage, only
In order to ensure the disinfection effect. At the same time, due to o-phthalaldehyde disinfectants and clothing.
After contact with the skin, it is easy to appear gray reaction, increase the difficulty of cleaning, so disinfection.
During the use of the agent need to strengthen their own skin protection efforts to minimize disinfection.
The spraying of the agent. At present, most hospitals in China are using traditional glutaraldehyde disinfectant
The disinfection of endoscopic equipment, although safe and reliable, but the disinfection time is longer, resulting in
The person cannot wait for a long time, suggesting that glutaraldehyde is completely used by o-phthalaldehyde disinfectant.
Sex replacement is just around the corner. The results of this study show that clinical disinfection by o-phthalaldehyde
After disinfection of endoscopes with the agent and glutaraldehyde disinfectant, a clinical pass rate of 100.0 percent was obtained,
It shows that the clinical disinfection effect of the two disinfectants is significant; the clinical effect of the two disinfectants is significant.
The difference was statistically significant compared to work efficiency and glutaraldehyde.
(P
<〇.〇5)。
To sum up, clinical use of phthalaldehyde endoscopic disinfection effect is significant, can be
Shorten the disinfection time, reduce toxicity, it is worth using.
[References]
[1] Zhou Xiaoliang, Li Wen. Comparative study on disinfection of digestive endoscopes with o-phthalaldehyde and glutaraldehyde
Investigate
[ J ] .
Chinese Journal of Microecology,
2013, 25 (4): 454 -4 5 6 .
[2] Lin Jiang, Gao Shan, Xu Haili, et al. o-phthalaldehyde, glutaraldehyde, chlorine-containing disinfectant
Comparison of disinfection effect and durability of mirrors [
J
]
.
Journal of Nurse Retraining
,
2〇13 ,
2 8
(
I
5 ):
The difference in work efficiency indicators such as appointment time and total disinfection was statistically significant.
(P
< 1414-1415.
〇.〇5)。
See Table
2。
[3]
Ou Yanni, Liang Zulan, Ye Wanhua, et al. Effect of o-phthalaldehyde on disinfection of endoscopes
Observation
[J].
Modern Clinical Nursing,
2012, 11 ( 1 ) : 31 -32.
RELATED INFORMATION









