Toxicological study of an o-phthalaldehyde disinfectant
Release time:
2022-07-18
To evaluate the safety and application value of an o-phthalaldehyde disinfectant (CidexOPA). Methods Different doses of the o-phthalaldehyde disinfectant (OPA) were used for acute oral toxicity test in mice, and the poisoning performance and death of mice in each group were observed and compared. Different doses of OPA and glutaraldehyde were used for micronucleus test of bone marrow polychromatic erythrocytes in mice, and the biological safety between OPA and glutaraldehyde was compared. Results The median lethal dose (LD) of CidexOPA to mice was> 6500mr,/kg (body weight). There was no difference in PCE micronucleus rate between the CidexOPA group and the glutaraldehyde group. Conclusion CidexOPA is non-toxic and has high safety in clinical application.
An o-phthalaldehyde disinfectant (CidexOPA) for clinical elimination
Toxic high-level disinfectant, currently mainly used in medical equipment immersion.
Disinfection, especially of endoscopes. Phase with other disinfectants currently used
It has a wide spectrum of sterilization, low concentration, short sterilization time, stimulation.
small, low corrosion characteristics, gradually widely used in clinical. This real
By testing its acute oral toxicity and bone marrow hypertrophic red in mice.
Effect of micronucleus formation in cells and with commonly used endoscopic disinfectant glutaraldehyde
To evaluate the safety of its clinical application.
1 Materials and methods
1.1 material
CidexOPA High Level Disinfectant (Johnson & Johnson) Main Ingredients
Light blue for o-phthalaldehyde (ortho-phthalaldehyde,OPA)
Color transparent liquid, ortho-phthalaldehyde content is 5500mg/L,
pH7.45. ICR Mouse [License No. SCXK(J)2007-
0003], purchased from Experimental Animal Center, Bethune Medical College, Jilin University,
Animals are kept in ordinary animal room, temperature 22 ~ C, relative humidity
50% 。
1.2 method
1.2.1 Acute oral toxicity test in miceSelect 18~22g small5O rats, randomly divided into 5 groups, each half male and half female, fasting before the test8h, can not help water. The dose of CidexOPA stock solution was 4.5, respectively,5.0, 5.5, 6.0 and 6.5 g/kg (body weight), according to 0.2mL/lOgSubsexual intragastric administration. After administration, normal feeding, continuous observation for 14 days, observationThe poisoning and death of mice in each group were observed and compared.
1.2.2 Mouse bone marrow polychromatic erythrocyte micronucleus test
Select 25
-
30g mice 60, randomly divided into 6 groups, each group of half male and half female, real
Fasting for 4 hours before the test can not help but water. CidexOPA experimental group dose
500, 2000 and 5000 mg/kg (body weight), the positive control group was given
Cyclophosphamide 40 mg/kg (body weight), the negative control group was given the same amount of steam
Distillate water gavage. Glutaraldehyde group was given 20 L glutaraldehyde 5000
mg/kg (body weight). The use of oral gavage 30h exposure method, that is, the first time.
After exposure, the animal was given once again 24 hours after exposure, and the animal was given 6 hours after the second administration.
Cervical vertebrae were sacrificed, calf serum flushed the femoral bone marrow cavity, and bone marrow was removed.
Lotion smear, formaldehyde fixed Giemsa staining after microscopic counting
Micronucleated cells contained in 1000 bone marrow polychromatic erythrocytes (PCE)
Number. And observe the normal red blood cell count (NCE), calculate the PCE/NCE
The ratio. Observation and comparison of mice in each group during the experiment eating, live
Number of moves, mental state.
2 Results
Results of acute oral toxicity test in 2.1 mice
CidexOPA according to the dose of gavage to mice, each dose group
The mice showed no adverse performance, and were observed for 14 days. During the process, the mice in each group were covered with hair.
Gloss, normal diet and activities, good mental state, no weight loss, complete
No mice died. Median lethal dose in mice> 6500 mg/kg
Weight.
Results of micronucleus test of bone marrow polychromatic erythrocytes in 2.2 mice
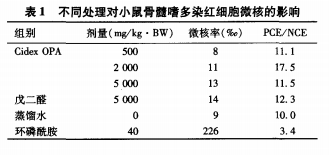
The micronucleus rate of mouse bone marrow polychromatic erythrocytes is shown in Table 1.
The rate (‰) is converted by square root and analyzed by variance, CidexOPA each.
The micronucleus rate of polychromatic erythrocytes in the dose group was not significantly higher than that in the negative control group.
The difference in sex (P>0.05),CidexOPA group and glutaraldehyde group mice
There was no significant difference in the micronucleus rate of polychromatic erythrocytes (P>
O.05), the difference between the positive control group and the negative control group is significant.
(P<0.01), the results showed that the three dose CidexOPA were not
It causes an increase in the incidence of micronuclei in mouse bone marrow polychromatic erythrocytes.
The PCE/NCE ratios were all greater than 0.1, demonstrating the effect of each test dose on bone
No significant inhibitory effect on the marrow, the experimental dose of CidexOPA on mice
Bone marrow polychromatic erythrocyte chromosomes were not damaged. experimental process
CidexOPA each group of mice was hair luster, diet, normal activity,
Good spirit. Glutaraldehyde group mice coat luster, normal diet, activity frequency
The rate is reduced, the spirit is still good. Cyclophosphamide group mice hair dull, drink
Food is acceptable, the frequency and range of activities are significantly reduced, and the spirit is poor.
3 Discussions
CidexOPA was first used in endoscopy in Canada in 1994
Disinfection, 1999 as a new type of high-efficiency chemical disinfectant through FDA
Certification, which achieves disinfection and sterilization through cross-linking, human membrane and other mechanisms.
With "]. According to the 2002 edition of the Disinfection Technical Regulations issued by the Ministry of Health.
Fan "chemical substances acute toxicity classification standard evaluation provisions, LD."....................................................................................
5000 mg/kg (body weight) is actually non-toxic, this experiment proves that ci-
dexOPA is a practically non-toxic grade.
In this experiment, mice bone marrow polychromatic erythrocytes were stained in each dose group.
There is no damage to the body, confirming the CidexOPA under the conditions of this experiment.
There is no mutagenic effect. CidexOPA group induced PCE and pentanediol
Although there is no difference between aldehydes, glutaraldehyde itself is inherently toxic and has a significant impact on medical
potential health hazards to personnel and patients. In the actual endoscopic decontamination
In order to meet the disinfection standard, glutaraldehyde needs a long time to soak. Example
Such as, 2100mg,/L of the OPA effect on the pollution of 10. cfu/ml cattle
The average killing time of mycobacteria was 6min, while 1.5% of
Aldehyde requires 30min J. CidexOPA as an efficient, economical,
Safe new disinfectant], with high clinical application value.
Previous Page
Next Page
RELATED INFORMATION









